The oxidation states and chemical environments of iron and zinc as potential indicators of brain tumour malignancy grade – preliminary results
Abstract
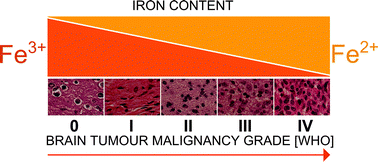
Despite the enormous advances in medicine, brain tumours are still among the lesser-known types of tumours and carry the worst prognoses. Transition metals are believed to play an essential role in carcinogenesis. The aim of this study was to determine differences in the average oxidation state and trends in the changes in the chemical environment of iron and zinc contained in healthy and neoplastic tissues of the human brain. For this purpose, X-ray Absorption Spectroscopy was used, which enables the study of disordered matter. The samples were taken intraoperatively and then immediately frozen to slow down chemical processes. Sixteen tumour samples with various malignancy grades were studied as well as one control sample. For each sample four to eight

 Please wait while we load your content...
Please wait while we load your content...