Selenoprotein P and selenoprotein M block Zn2+-mediated Aβ42 aggregation and toxicity†
Abstract
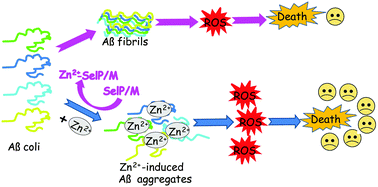
Aggregation and cytotoxicity of the amyloid-β (Aβ)
- This article is part of the themed collection: Metallomics in China
Maintenance work is planned from 09:00 BST to 12:00 BST on Saturday 28th September 2024.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a
College of Life Sciences, Shenzhen Key Laboratory of Microbial Genetic Engineering, Shenzhen University, Shenzhen 518060, China
E-mail:
jzni@szu.edu.cn
Tel: +86-755-26536629
b
Department of Marine Biology, Shenzhen Key Laboratory of Marine Biotechnology and Ecology, Shenzhen 518060, China
E-mail:
liuqiong@szu.edu.cn
Tel: +86-755-26534152
Aggregation and cytotoxicity of the amyloid-β (Aβ)

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
