Thermodynamics and solvation dynamics of BIV TAR RNA–Tat peptide interaction†
Abstract
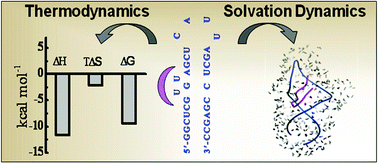
The interaction of the trans-activation responsive (TAR) region of bovine immunodeficiency virus (BIV) RNA with the Tat peptide is known to play important role in viral replication. Despite being thoroughly studied through a structural point of view, the nature of binding between BIV TAR RNA and the BIV Tat peptide requires information related to its thermodynamics and the nature of hydration around the TAR–Tat complex. In this context, we carried out the thermodynamic study of binding of the Tat peptide to the BIV TAR RNA hairpin through different calorimetric and spectroscopic measurements. Fluorescence titration of 2-aminopurine tagged BIV TAR RNA with the Tat peptide gives their binding affinity. The isothermal titration calorimetric experiment reveals the enthalpy of binding between BIV TAR RNA and the Tat peptide to be largely exothermic with the value of −11.7 (SEM 0.2) kcal mol−1. Solvation dynamics measurements of BIV TAR RNA having 2-AP located at the bulge region have been carried out in the absence and presence of the BIV Tat peptide using the time correlated single photon counting technique. The solvent cage around the Tat binding site of RNA appears to be more rigid in the presence of the Tat peptide as compared to the free RNA. The displacement of solvent and ions on RNA due to peptide binding influences the entropic contributions to the total binding energy.

 Please wait while we load your content...
Please wait while we load your content...