In situ laser micropatterning of proteins for dynamically arranging living cells
Abstract
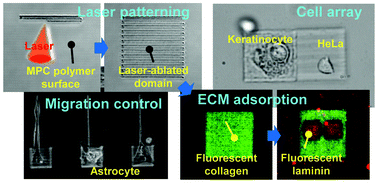
This study shows the modification of the surface of
* Corresponding authors
a
Center for Interdisciplinary Science, National Chiao Tung University, 1001 Ta-Hsueh Rd., Hsinchu 30010, Taiwan
E-mail:
okano@nctu.edu.tw
b Graduate School of Materials Science, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan
c
Institute of Biophotonics, National Yang-Ming University, No.155, Sec. 2, Linong St., Beitou District, Taipei 112, Taiwan
E-mail:
fjkao@ym.edu.tw
d Kansei Fukushi Research Institute, Tohoku Fukushi University, 6-149-1 Kunimigaoka, Aoba-ku, Sendai 989-3201, Japan
e Toin University of Yokohama, 1614 Kurogane-cho, Aoba-ku, Yokohama 225-8503, Japan
f
Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung University, 1001 Ta-Hsueh Rd., Hsinchu 30010, Taiwan
E-mail:
masuhara@masuhara.jp
g Waseda Institute for Advanced Study, Waseda University, 1-6-1 Nishiwaseda, Shinjuku-ku, Tokyo 169-8050, Japan
This study shows the modification of the surface of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
K. Okano, A. Matsui, Y. Maezawa, P. Hee, M. Matsubara, H. Yamamoto, Y. Hosokawa, H. Tsubokawa, Y. Li, F. Kao and H. Masuhara, Lab Chip, 2013, 13, 4078 DOI: 10.1039/C3LC50750E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
