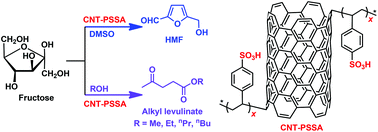
A series of sulfonic acid-functionalized carbon materials (C–SO3H), including poly(p-styrenesulfonic acid)-grafted carbon nanotubes (CNT-PSSA), poly(p-styrenesulfonic acid)-grafted carbon nanofibers (CNF-PSSA), benzenesulfonic acid-grafted CMK-5 (CMK-5-BSA), and benzenesulfonic acid-grafted carbon nanotubes (CNT-BSA), have been studied for fructose dehydration to 5-hydroxymethylfurfural (HMF) and fructose alcoholysis to alkyl levulinate. A study for optimizing the reaction conditions such as the catalyst loading, the reaction time, and the temperature has been performed. Under the optimal conditions, high HMF and ethyl levulinate yields of up to 89% and 86%, respectively, are obtained. The catalytic activities of C–SO3H for the conversions of fructose into both HMF and ethyl levulinate follow the order of their acid strength. The relationship between the catalytic activity and acid density of C–SO3H shows a linear correspondence in the fructose dehydration to HMF. The facile separation, ease of recovery, and high thermal stability make the developed C–SO3H efficient and environment-friendly catalytic materials for transforming biomass carbohydrate into fine chemicals.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...