Reactions of p-coumaryl alcohol model compounds with dimethyl carbonate. Towards the upgrading of lignin building blocks†
Abstract
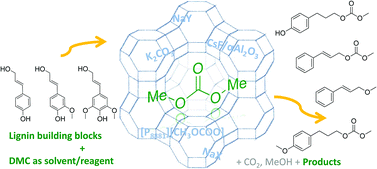
Cinnamyl alcohol 1 and 4-(3-hydroxypropyl)phenol 2, two compounds resembling the lignin building block p-coumaryl alcohol, can be selectively transformed into different products by catalytic methodologies based on dimethyl carbonate (DMC) as a green solvent/reagent. Selectivity can be tuned as a function of the reaction temperature and of the nature of the catalyst. Basic catalysts such as K2CO3, trioctylmethylphosphonium methylcarbonate ([P8881][CH3OCOO]), and CsF/αAl2O3 promote selective transesterification of the aliphatic hydroxyl group at 90 °C. However, amphoteric solids such as alkali metal-exchanged faujasites, NaX and NaY, selectively yield the corresponding alkyl ethers at higher temperatures (165–180 °C). The phenolic hydroxyl group of 2 can be methylated similarly with the faujasites at high temperatures. This preliminary screening for selectivity illustrates reactivity trends and delineates some of what might be among the most promising synthetic pathways to upgrade lignin-derived chemical building blocks.
- This article is part of the themed collection: Green Solvents

 Please wait while we load your content...
Please wait while we load your content...