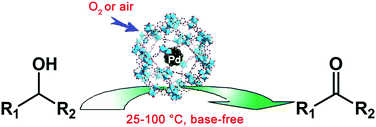
Highly dispersed palladium nanoparticles were deposited on an acidic MOF (MIL-101) by using a simple colloidal method. The resulting Pd/MIL-101 catalyst was highly active in the liquid-phase aerobic oxidation of a wide range of alcohols including benzyl, allylic, aliphatic and heterocyclic alcohols as well as diols, affording the desired oxidation products in high yields under base-free and mild conditions. The catalyst was shown to be able to efficiently catalyze aerobic oxidation even at ambient temperature using air instead of pure O2. The solvent-free oxidation of benzyl alcohol gave a remarkably high turnover frequency (TOF) of approximately 16 900 h−1. However, the catalytic activity was significantly suppressed when ethylenediamine was grafted on the uncoordinated Cr sites of the MIL-101 support, which suggests that the open Cr sites might play an important role in promoting the oxidation of alcohols in the present catalytic system.