Linking thermodynamics and kinetics to assess pathway reversibility in anaerobic bioprocesses†
Abstract
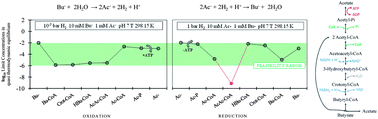
The on-going research towards sustainable fuel production entails the improvement of the microbial catalysts involved. The possible reversibility of specific anaerobic catabolic reactions opens up a range of possibilities for the development of novel reductive bioprocesses. These reductive biohydrogenation pathways enable production of high energy density chemicals of interest as biofuels such as alcohols and long chain fatty acids. Anaerobic bioprocesses take place under energy scarcity conditions due to the absence of strong electron acceptors such as oxygen, and provide metabolic pathways towards these energy dense (reduced) chemicals. Metabolic reactions take place very close to thermodynamic equilibrium with minimum energy dissipation and consequently, environmental changes in product and substrate concentrations can easily reverse the driving force of the chemical reaction catalysed. The objective of this work is to investigate the potential reversibility of specific anaerobic pathways of interest. The thermodynamics of the different steps in biochemical pathways are analysed and combined with assumptions concerning kinetic and physiological constraints to evaluate if pathways are potentially reversible by imposing changes in process conditions. The results suggest that (i) in homoacetogenesis they may operate in both reductive and oxidative directions depending on the hydrogen partial pressure in the system, (ii) acetate reduction to butyrate with hydrogen is not feasible, but no biochemical bottlenecks are apparent in butyrate production from acetate with ethanol or lactate as electron donors, (iii) the reduction of short chain to longer chain fatty acids with ethanol as the electron donor appears thermodynamically and kinetically feasible, and (iv) alcohol production from the corresponding fatty acids (e.g. ethanol from acetate) was found to require proton translocations at specific sites in the biochemical pathways in order to compensate for the ATP required for phosphatation of acetate and to enable energy harvesting. Overall, the methodology proposed here allows for analysing the potential reversibility of catabolic pathways and therewith contributes to the development of efficient and reliable anaerobic bioprocesses for the production of biofuels and chemicals.

 Please wait while we load your content...
Please wait while we load your content...