Activation of a (cyclooctadiene) rhodium(i) complex supported by a chiral ferrocenyl phosphine thioether ligand for hydrogenation catalysis: a combined parahydrogen NMR and DFT study†
Abstract
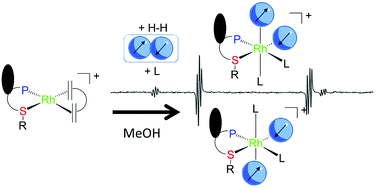
The reaction of [RhCl(P,StBu)(COD)] (1) or [Rh(P,StBu)(COD)]BF4 (2) where (P,StBu) is CpFe[η5-1,2-C5H3(PPh2)(CH2StBu)] with H2 in MeOH gives rise to COD hydrogenation and formation of a solvent-stabilized product. The formation of hydride species cannot be observed in view of a very rapid H/D exchange between H2 and the solvent. Introduction of pyridine or acetonitrile slows down this exchange process and allows observation of diastereometric dihydride complexes, [Rh(P,StBu)(H)2(L)2]+, the stereochemistry of which was fully elucidated. The hydride site exchange rates have been derived from EXSY NMR experiments and used, with assistance from DFT calculation, to elucidate the isomerization and site exchange mechanisms.

 Please wait while we load your content...
Please wait while we load your content...