Stabilization of diborane(4) by transition metal fragments and a novel metal to π Dewar–Chatt–Duncanson model of back donation†
Abstract
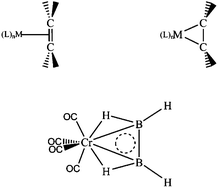
The feasibility of using transition metal fragments to stabilize B2H4 in planar configuration by donating 2 electrons to the boron moiety is investigated. Building upon the existing theoretical and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bond.
B double bond.

 Please wait while we load your content...
Please wait while we load your content...