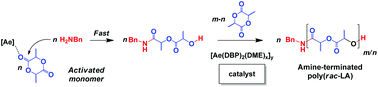
Calcium and strontium metals react with Hg(C6F5)2 and 2,4-di-tert-butylphenol (H-DBP) in tetrahydrofuran (THF) and 1,2-dimethoxyethane (DME) to give [Ca(DBP)2(THF)4] (1), [Ca2(DBP)4(DME)4(μ-DME)] (2), [Sr3(μ-DBP)6(THF)6] (3), and [Sr2(DBP)(μ-DBP)3(DME)3] (4). Compound 1 is a six coordinate trans-octahedral monomer, whereas in binuclear 2 two seven-coordinate Ca centres are bridged by a DME ligand. In 3 a central Sr is connected by three bridging DBP groups to each of two terminal Sr(THF)3 moieties, all metal atoms being six coordinate. Compound 4 has one six- and one seven-coordinate Sr, bridged by three DBP ligands, the former Sr also having a terminal DBP and a bidentate DME ligand and the latter two DME ligands. Complexes 2 and 4 act as ring-opening polymerisation (ROP) catalysts for the benzyl alcohol or benzylamine co-initiated ROP rac-lactide forming atactic alcohol- or amine-terminated polylactide H-[PLA]-XBn (X = O or NH) with reasonable control of molecular weight via an activated monomer propagation mechanism. Kinetic studies for BnNH2 found the unusual rate expression −d[LA]/dt = kp(Ae)[2 or 4]0[rac-LA]2[BnNH2]02.5 (kp(Ca) ≈ 1.7 × kp(Sr)). Preliminary studies suggest that [Y(DBP)3(THF)2] also catalyses amine or alcohol co-initiated ROP by an activated monomer mechanism without loss of a phenoxide ligand.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...