Synthesis, structure, magnetic properties and theoretical calculations of methoxy bridged dinuclear iron(iii) complex with hydrazone based O,N,N-donor ligand†
Abstract
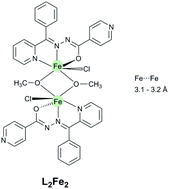
Biomimetic complexes are artificially engineered molecules that aim to reduce the structural complexity of biological systems in order to unveil the key electronic and structural factors relevant to a protein's function. In this work, a novel coordination compound (L22Fe22) which mimics non-heme binuclear

 Please wait while we load your content...
Please wait while we load your content...