DFT study of the mechanism for methane hydroxylation by soluble methane monooxygenase (sMMO): effects of oxidation state, spin state, and coordination number†
Abstract
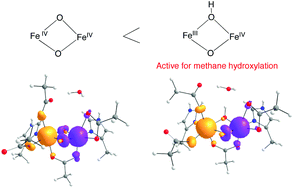
The exact structure of the active site of intermediate Q, the methane-oxidizing species of soluble methane monooxygenase (sMMO), and the reaction mechanism of Q with methane molecule are still not fully clear. To gain further insights into the structure and reaction mechanism, five diiron models of Q that differ in shape, oxidation state, spin state, and coordination number of the two iron centers are studied. Different mechanisms in different spin states were explored. Density functional theory (DFT) calculations show that FeIIIFeIV(μ-O)(μ-OH) is more reactive than FeIV2(μ-O)2 in the oxygen-rich environment and that the reactivity of the active core of sMMO-Q is not enhanced by converting its oxo bridge into a terminal ligand. A four-coordinated diiron model is the most effective for methane hydroxylation. Both radical and non-radical intermediates are involved in the reactions for the four-coordinated diiron model.

 Please wait while we load your content...
Please wait while we load your content...