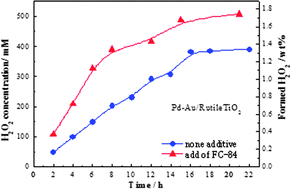
Synthesis of H2O2 by oxidation of H2 with air on Au–Pd/TiO2 was studied and it was found that H2 conversion and H2O2 selectivity were strongly affected by the crystal phase of the TiO2 support for Au–Pd. At 0.1 MPa, brookite type TiO2 was active for H2O2 synthesis because of high H2 conversion (40%) and selectivity (67%). In contrast, rutile type TiO2 showed high H2 conversion but low selectivity. With increasing H2 pressure, H2O2 selectivity increased on Au–Pd/rutile TiO2 and the H2O2 yield became much higher on the catalyst supported on rutile TiO2 than those on brookite or anatase at 1.0 MPa. The effects of fluorinated hydrocarbon addition to reaction media were also studied, and it was found that the addition of a small amount of fluorinated hydrocarbon was effective for increasing H2O2 productivity. The optimized fluorinated hydrocarbon was CF3(CF2)5CF3, and the amount was 1/10 (7.5 ml) of the reaction media (75 ml). The H2O2 formation rate of 3.78 mmol h−1 was achieved under the optimized conditions, and the concentration of 1.8 wt% H2O2 was achieved after 20 h.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...