Catalytic urea hydrolysis in the selective catalytic reduction of NOx: catalyst screening and kinetics on anatase TiO2 and ZrO2
Abstract
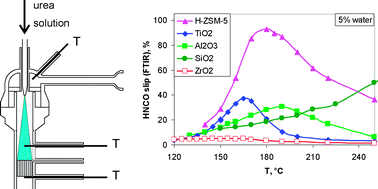
The catalytic hydrolysis of urea was investigated under conditions relevant for the selective catalytic reduction of NOx (urea-SCR). The hydrolysis activities of the tested catalysts coated on cordierite monoliths were in the order ZrO2 > TiO2 > Al2O3 > H-ZSM-5 > SiO2. A comparison with isocyanic acid (HNCO) hydrolysis on the same catalysts showed that urea decomposition was much slower than HNCO hydrolysis; hence, catalytic urea thermolysis into NH3 and HNCO is likely to be the rate-determining step in urea decomposition. Interestingly, a different order of catalyst activities was found in water-free experiments on urea thermolysis: TiO2 > H-ZSM-5 ≈ Al2O3 > ZrO2 > SiO2. The widely accepted reaction pathway for urea decomposition, namely urea thermolysis followed by HNCO hydrolysis, seems to be valid on all the tested catalysts except ZrO2: The high urea hydrolysis activity of the ZrO2 catalyst compared to its low urea thermolysis activity suggested a different reaction pathway, in which water directly attacks adsorbed urea rather than adsorbed HNCO.

 Please wait while we load your content...
Please wait while we load your content...