Asymmetric Marcus–Hush theory for voltammetry
Abstract
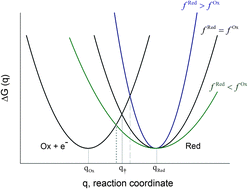
The current state-of-the-art in modeling the rate of electron transfer between an electroactive species and an electrode is reviewed. Experimental studies show that neither the ubiquitous Butler–Volmer model nor the more modern symmetric Marcus–Hush model are able to satisfactorily reproduce the experimental voltammetry for both solution-phase and surface-bound redox couples. These experimental deviations indicate the need for revision of the simplifying approximations used in the above models. Within this context, models encompassing asymmetry are considered which include different vibrational and solvation force constants for the electroactive species. The assumption of non-adiabatic electron transfer is also examined. These refinements have provided more satisfactory models of the electron transfer process and they enable us to gain more information about the microscopic characteristics of the system by means of simple electrochemical measurements.

 Please wait while we load your content...
Please wait while we load your content...