Coordination chemistry beyond Werner: interplay between hydrogen bonding and coordination†
Abstract
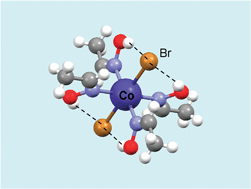
A tutorial review is presented dealing with the role of hydrogen bonding in coordination compounds. Examples are given for important intramolecular hydrogen bonding, and stabilising unusual coordination geometries, or reactive species. Also examples are discussed for intermolecular H-bonds between
- This article is part of the themed collection: Alfred Werner Nobel Prize 100 year celebration

 Please wait while we load your content...
Please wait while we load your content...