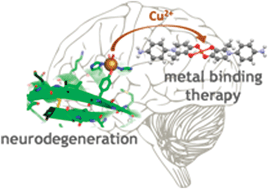
Neurodegenerative diseases are capturing the world's attention as being the next set of diseases we must tackle collectively. Not only are the patients experiencing gradual cognitive and physical decline in most cases, but these diseases are fatal with no prevention currently available. As these diseases are progressive, providing care and symptom treatment for the ageing population is becoming both a medical and a financial challenge. This review discusses how Werner coordination chemistry plays a role in three diseases – those of Alzheimer's, Parkinson's, and prions. Metal ions are considered to be involved in these diseases in part via their propensity to cause toxic aggregation of proteins. First, the coordination of metal ions, with emphasis on copper(II), to metalloproteins that are hallmarks of these diseases – amyloid β, α-synuclein, and prion, respectively – will be discussed. We will present the current understanding of the metal coordination environments created by the amino acids of these proteins, as well as metal binding affinity. Second, a diverse set of examples of rationally designed metal chelators to outcompete this deleterious binding will be examined based on coordination mode and affinity toward bio-relevant metal ions. Overall, this review will give a general overview of protein and metal chelator coordination environments in neurodegenerative diseases.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...