Methane storage capabilities of diamond analogues†
Abstract
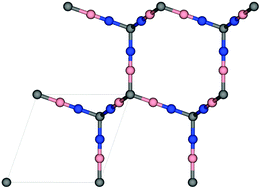
Methane can be an alternative fuel for vehicular usage provided that new porous materials are developed for its efficient adsorption-based storage. Herein, we search for materials for this application within the family of diamond analogues. We used density functional theory to investigate structures in which tetrahedral C atoms of diamond are separated by –CC– or –BN– groups, as well as ones involving substitution of tetrahedral C atoms with Si and Ge atoms. The adsorptive and diffusive properties of methane are studied using classical molecular simulations. Our results suggest that the all-carbon structure has the highest volumetric methane uptake of 280 VSTP/V at p = 35 bar and T = 298 K. However, it suffers from limited methane diffusion. Alternatively, the considered Si and Ge-containing analogies have fast diffusive properties but their adsorption is lower, ca. 172–179 VSTP/V, at the same conditions.

 Please wait while we load your content...
Please wait while we load your content...