Intramolecular halogen–halogen bonds?†
Abstract
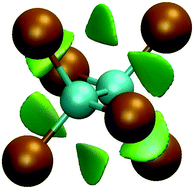
By analysing the properties of the electron density in the structurally simple perhalogenated ethanes, X3C–CY3 (X, Y = F, Cl), a previously overlooked non-covalent attraction between
Maintenance work is planned from 09:00 BST to 12:00 BST on Saturday 28th September 2024.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a Institut de Química Computational i Catàlisi and Departament de Química, Universitat de Girona, Campus Montilivi, ES-17071 Girona, Spain
b
Laboratory for Instruction in Swedish, Department of Chemistry, University of Helsinki, FI-00014 Helsinki, Finland
E-mail:
mikael.johansson@iki.fi
c
Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain
E-mail:
marcel.swart@icrea.cat
By analysing the properties of the electron density in the structurally simple perhalogenated ethanes, X3C–CY3 (X, Y = F, Cl), a previously overlooked non-covalent attraction between

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. P. Johansson and M. Swart, Phys. Chem. Chem. Phys., 2013, 15, 11543 DOI: 10.1039/C3CP50962A
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
