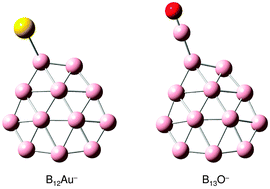
We report a photoelectron spectroscopy and density-functional theory study of the B12Au− and B13O− clusters and their neutrals, which are shown to be six π electron aromatic compounds between the quasi-planar all-boron B12 benzene-analogue and a monovalent Au or BO ligand. Electron affinities of B12Au and B13O are measured to be 3.48 ± 0.04 and 3.90 ± 0.04 eV, respectively. Structural searches are performed for B12Au− and B13O−, which are compared with the isovalent B12H− cluster. The global minima of B12Au− and B13O− both feature an almost intact B12 cluster with the Au and BO ligands bonded to its periphery, respectively. For B12Au−, a low-lying isomer is also identified, which is only 0.4 kcal mol−1 above the global minimum, in agreement with the experimental observation of a weakly populated isomer in the cluster beam of B12Au−. These aromatic compound clusters provide new examples for the Au/H isolobal analogy and the boronyl (BO) chemistry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...