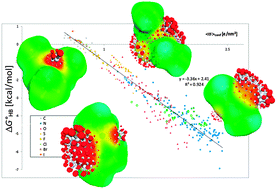
In this work, experimental hydrogen-bond (HB) enthalpies measured in previous works for a wide range of acceptor molecules in dilute mixtures of 4-fluorophenol in non-polar solvents are quantified from COSMO polarisation charge densities σ of HB acceptors (HBA). As well as previously demonstrated for quantum chemically calculated HB enthalpies, a good correlation of the experimental data with the polarisation charge densities is observed, covering an extended range of HBA (O, N, S, π systems and halogens) ranging from very weak to strong hydrogen bonds. Furthermore, for the first time, a quantitative analysis of experimental HB entropies is performed for such a chemical diversity of HBA. A good quantification of these entropies is achieved using the polarisation charge density σ as a descriptor in combination with the logarithm of a directional partition function ΩHB. This partition function covers the directional and multiplicity entropy of HBA and is based on the σ-proportional HB enthalpy expression taken from COSMO-RS. As a result, the experimental HB enthalpies and free energies of the ∼300 HB complexes are quantified with an accuracy of ∼2 kJ mol−1 based on COSMO polarisation charge densities.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...