Impact of long-range van der Waals forces on chiral recognition in a Cinchona alkaloid chiral selector system†
Abstract
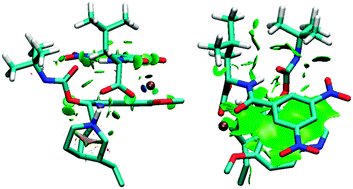
Singly-charged complexes of (8S,9R)-tert-butylcarbamoylquinine (tBuCQN), N-3,5-dinitrobenzoyl-(S,R)-leucine (DNB-S/
Maintenance work is planned from 09:00 BST to 12:00 BST on Saturday 28th September 2024.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a
Charles University in Prague, Faculty of Science, Department of Organic Chemistry, Hlavova 8, 12843 Prague 2, Czech Republic
E-mail:
petr.milko@weizmann.ac.il, jana.roithova@natur.cuni.cz
Tel: +420-221-951-322
b
Department of Chemistry & Biochemistry, The University of Texas at Arlington, 700 Planetarium Pl., Arlington, TX 76019-0065, USA
E-mail:
kschug@uta.edu
Tel: +1-817-272-3541
c
Regional Centre of Advanced Technologies and Materials, Department of Analytical Chemistry, Faculty of Science, Palacky University, 17. listopadu 12, 77146 Olomouc, Czech Republic
E-mail:
karel.lemr@upol.cz
Tel: +420-585-634-415
Singly-charged complexes of (8S,9R)-tert-butylcarbamoylquinine (tBuCQN), N-3,5-dinitrobenzoyl-(S,R)-leucine (DNB-S/

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
P. Milko, J. Roithová, K. A. Schug and K. Lemr, Phys. Chem. Chem. Phys., 2013, 15, 6113 DOI: 10.1039/C3CP44444A
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
