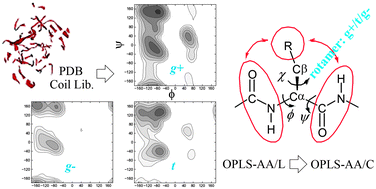
The local conformational (ϕ, ψ, χ) preferences of amino acid residues remain an active research area, which are important for the development of protein force fields. In this perspective article, we first summarize spectroscopic studies of alanine-based short peptides in aqueous solution. While most studies indicate a preference for the PII conformation in the unfolded state over α and β conformations, significant variations are also observed. A statistical analysis from various coil libraries of high-resolution protein structures is then summarized, which gives a more coherent view of the local conformational features. The ϕ, ψ, χ distributions of the 20 amino acids have been obtained from a protein coil library, considering both backbone and side-chain conformational preferences. The intrinsic side-chain χ1 rotamer preference and χ1-dependent Ramachandran plot can be generally understood by combining the interaction of the side-chain Cγ/Oγ atom with two neighboring backbone peptide groups. Current all-atom force fields such as AMBER ff99sb-ILDN, ff03 and OPLS-AA/L do not reproduce these distributions well. A method has been developed by combining the ϕ, ψ plot of alanine with the influence of side-chain χ1 rotamers to derive the local conformational features of various amino acids. It has been further applied to improve the OPLS-AA force field. The modified force field (OPLS-AA/C) reproduces experimental 3J coupling constants for various short peptides quite well. It also better reproduces the temperature-dependence of the helix–coil transition for alanine-based peptides. The new force field can fold a series of peptides and proteins with various secondary structures to their experimental structures. MD simulations of several globular proteins using the improved force field give significantly less deviation (RMSD) to experimental structures. The results indicate that the local conformational features from coil libraries are valuable for the development of balanced protein force fields.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...