Multi-electron redox reaction of an organic radical cathode induced by a mesopore carbon network with nitroxide polymers†
Abstract
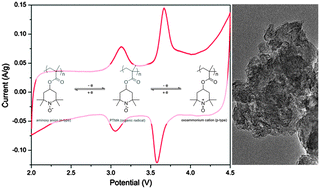
An organic radical based composite cathode comprised of poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl methacrylate) (PTMA)–Ketjenblack was developed by a simple solvent-less electrode fabrication method. The composite cathode demonstrated a two-electron redox reaction of PTMA that is from an aminoxy anion (n-type) via a radical to an oxoammonium cation (p-type) with the corresponding redox potential at 2.8–3.1 V and 3.5–3.7 V vs. Li/Li+ when evaluated in lithium half cells. Moreover, the PTMA–Ketjenblack composite electrode exhibits fast electrode reaction kinetics and an enhanced solid electrolyte interface by cyclic voltammetry and electrochemical impedance spectroscopy measurements. These improved electrochemical properties contribute to increased capacity (300 mA h g−1), a high rate (50% capacity retention after 100 C rate excursions) and a long cycle life in the cell performance evaluations. Morphological and compositional characterization indicates a unique mesopore network of Ketjenblack with the PTMA matrix, which highly facilitates the interaction between the conductive media and the radical species, resulting in the performance enhancement of the PTMA–Ketjenblack composite cathode.

 Please wait while we load your content...
Please wait while we load your content...