Non-purged voltammetry explored with AGNES†
Abstract
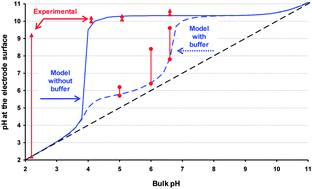
The reduction of oxygen increases pH in the surroundings of an electrode. A theoretical model estimates the steady-state pH profile from the surface of the electrode up to the bulk solution. A very simple formula predicts that, in non-deaerated solutions, for bulk pH-values between 4.0 and 10.0, the corresponding surface pH could be as high as 10.3, regardless of the thickness of the diffusion layer and composition of the sample (except if it has a buffering capacity). For bulk pH lower than 3.0 or higher than 10, pH increases are negligible. Less steep pH-profiles are obtained using buffers (such as MOPS 0.01 M or MES 0.01 M). The change in surface pH modifies the local speciation and hinders the standard interpretation of voltammetric responses. The electroanalytical technique Absence of Gradients and Nernstian Equilibrium Stripping (AGNES), implemented with Screen Printed Electrodes (SPE), provides experimental insights into this phenomenon. AGNES probes the free metal concentration at the electrode surface, from which the surface pH can be estimated for systems of known composition. These estimations agree with the theoretical model for the assayed systems. Additionally, the quantification of the bulk free Zn2+ and Cd2+ concentrations with specific modifications of AGNES for non-purged synthetic solutions is discussed. In general, more accurate determinations of the bulk free metal concentrations in non-purged solutions are expected: (i) when the calibration is performed in a medium where the pH increase induces similar changes in the surface free metal concentration and in the sample solution and (ii) when the system is more buffered.

 Please wait while we load your content...
Please wait while we load your content...