Determination of the mass-transport properties of vanadium ions through the porous electrodes of vanadium redox flow batteries
Abstract
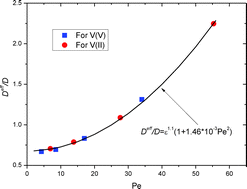
This work is concerned with the determination of two critical constitutive properties for mass transport of ions through porous electrodes saturated with a liquid electrolyte solution. One is the effective diffusivity that is required to model the mass transport at the representative element volume (REV) level of porous electrodes in the framework of Darcy's law, while the other is the pore-level mass-transfer coefficient for modeling the mass transport from the REV level to the solid surfaces of pores induced by redox reactions. Based on the theoretical framework of mass transport through the electrodes of vanadium redox flow batteries (VRFBs), unique experimental setups for electrochemically determining the two transport properties by measuring limiting current densities are devised. The effective diffusivity and the pore-level mass-transfer coefficient through the porous electrode made of graphite felt, a typical material for VRFB electrodes, are measured at different electrolyte flow rates. The correlation equations, respectively, for the effective diffusivity and the pore-level mass-transfer coefficient are finally proposed based on the experimental data.

 Please wait while we load your content...
Please wait while we load your content...