DNA photoreacts by nucleobase ring cleavage to form labile isocyanates†
Abstract
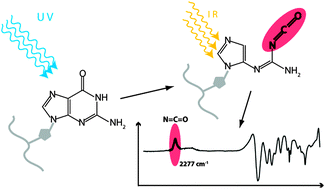
Differential infrared absorption spectroscopy was used to study the formation of isocyanates and further photo-products in the oligonucleotides dG10, dC10 and dT10 and in their mononucleosides by ultraviolet light at 266 nm. We find that α-cleavage takes place in oligonucleotides and mononucleosides both in films and in solution. The very intense and spectrally isolated isocyanate (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) asymmetric stretch vibration at 2277 cm−1 is used as a spectroscopic marker for detection of the photo-product. The band disappears upon reaction with small amounts of water vapour as expected for isocyanates. Quantum yields for isocyanate formation by nucleobase ring cleavage in the α-position to the carbonyl group are ∼5 × 10−5 in the mononucleosides and up to 5 × 10−4 in the oligonucleotides. In the mixed oligonucleotides dG10/dC10 and dA10/dT10 the quantum yield of α-cleavage drops by a factor of 10 compared to the single oligonucleotides. Implications for DNA repair and photo-induced DNA–protein cross-linking via isocyanate reaction with NH2 groups of amino acids are discussed.
O) asymmetric stretch vibration at 2277 cm−1 is used as a spectroscopic marker for detection of the photo-product. The band disappears upon reaction with small amounts of water vapour as expected for isocyanates. Quantum yields for isocyanate formation by nucleobase ring cleavage in the α-position to the carbonyl group are ∼5 × 10−5 in the mononucleosides and up to 5 × 10−4 in the oligonucleotides. In the mixed oligonucleotides dG10/dC10 and dA10/dT10 the quantum yield of α-cleavage drops by a factor of 10 compared to the single oligonucleotides. Implications for DNA repair and photo-induced DNA–protein cross-linking via isocyanate reaction with NH2 groups of amino acids are discussed.

 Please wait while we load your content...
Please wait while we load your content...