Single molecule recordings of lysozyme activity
Abstract
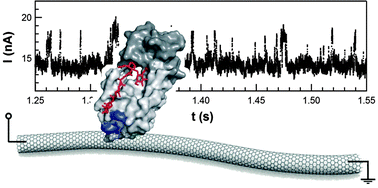
Single molecule bioelectronic circuits provide an opportunity to study chemical kinetics and kinetic variability with bond-by-bond resolution. To demonstrate this approach, we examined the catalytic activity of T4 lysozyme processing

 Please wait while we load your content...
Please wait while we load your content...