Destabilization of noble-gas hydrides by a water environment: calculations for HXeOH@(H2O)n, HXeOXeH@(H2O)n, HXeBr@(H2O)n, HXeCCH@(H2O)n
Abstract
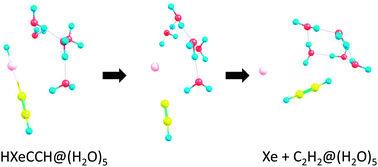
HNgY molecules are chemically-bound compounds of a noble-gas atom (Ng) with a hydrogen and with an electronegative group Y. There is considerable current interest in the stability of these species in different types of media. The kinetic stability of several compounds, HXeOH, HXeOXeH, HXeBr and HXeCCH, in water clusters is explored by ab initio calculations. It is found that the kinetic stability of the compounds is reduced by the water environment, generally falling off with the number of

 Please wait while we load your content...
Please wait while we load your content...