Quantification of photocatalytic hydrogen evolution†
Abstract
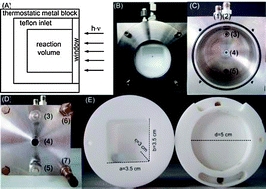
A new photoreactor with defined irradiation geometry was developed and tested for the water reduction reaction using carbon nitride (“C3N4”) as a photocatalyst. The hydrogen evolution rate was investigated with a sun simulator (I = 1000 W m−2) in two different operation modes: circulation and stirring of the

 Please wait while we load your content...
Please wait while we load your content...