Two viable three-dimensional carbon semiconductors with an entirely sp2 configuration
Abstract
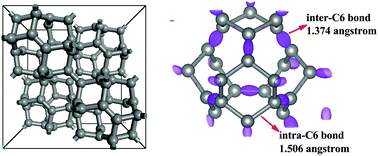
Using the first-principles method, we investigate the energetic stability, dynamic stability and electronic properties of two viable three-dimensional (3D) carbon semiconductors with an entirely sp2 configuration, sp2-diamond and cubic-graphite. Both allotropes are more stable than the previously proposed K4-carbon and T-carbon and were confirmed to be dynamically stable. Interestingly, sp2-diamond and cubic-graphite behave as semiconductors, which is contrary to previously proposed all-sp2 metallic carbons. sp2-Diamond is a semiconductor with a direct band gap of 1.66 eV and cubic-graphite is an indirect semiconductor with a band gap of 2.89 eV. Further studies show that both sp2-diamond and cubic-graphite possess structural all-sp2 configurations but not electronic sp2 hybridizations. The very low densities and entirely sp2 configurations of sp2-diamond and cubic-graphite can be potentially applied in hydrogen-storage, photocatalysts and molecular sieves.

 Please wait while we load your content...
Please wait while we load your content...