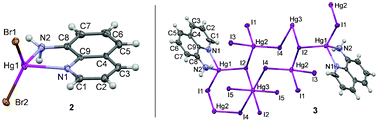
To investigate the effect of counterions on crystalline networks, we have designed and synthesized a series of HgII complexes, [Hg(8-aq)Cl2] (1), [Hg(8-aq)Br2] (2) and [Hg5(8-aq)2I10]n (3), via a reaction between 8-aminoquinoline (8-aq) with HgX2 (X = Cl, Br, I). They have been characterized by elemental analyses, IR, 1H- and 13C-NMR spectroscopy and two of the compounds (X = Br and I) have been structurally determined by single crystal X-ray diffraction analysis. These studies revealed that compound 2 is mononuclear with the metal centre exhibiting a distorted tetragonal geometry while compound 3 is a one-dimensional coordination polymer where the bridging iodide ligands and the metal centres exhibit distorted tetragonal and octahedral geometries. The non-covalent interactions responsible for the crystalline network of 2 and 3 have been studied using theoretical methods by means of dispersion-corrected density functional theory (DFT-D) calculations. The binding energies associated with the different non-covalent interactions indicate hydrogen bonds, stacking and X⋯X interactions govern the network formation of complexes 2 and 3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...