Oxidative 1,2-difunctionalization of activated alkenes with benzylic C(sp3)–H bonds and aryl C(sp2)–H bonds†
Abstract
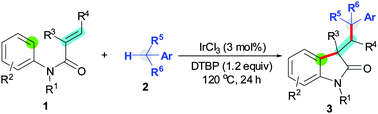
DTBP (di-tert-butyl peroxide) is utilized to mediate oxidative 1,2-difunctionalization of activated alkenes with an aryl C(sp2)–H bond and a benzylic C(sp3)–H bond for the synthesis of functionalized oxindoles. This reaction is a new organomediated strategy for alkene difunctionalization facilitated by Lewis acids.

 Please wait while we load your content...
Please wait while we load your content...