Highly enantioselective synthesis of chiral 7-ring O- and N-heterocycles by a one-pot nitro-Michael–cyclization tandem reaction†
Abstract
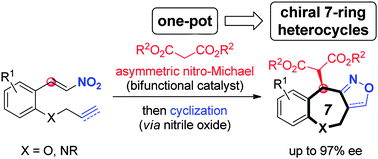
A concise enantioselective approach to synthesise medium-sized 7-ring O- and N-heterocycles has been developed. The synthetic strategy relies on an organocatalytic nitro-Michael–nitrile oxide cycloaddition tandem reaction, leading to the corresponding chiral benzoxe- and benzazepine derivatives containing an additional fused dihydroisoxazoline ring in good yields and excellent enantioselectivities (up to 97% ee).

 Please wait while we load your content...
Please wait while we load your content...