Advances in nucleophilic phosphine catalysis of alkenes, allenes, alkynes, and MBHADs
Abstract
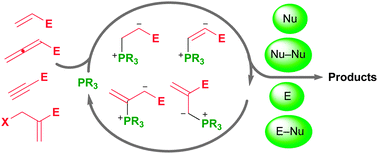
In nucleophilic phosphine catalysis, tertiary phosphines undergo conjugate additions to activated carbon–carbon multiple bonds to form β-phosphonium enolates, β-phosphonium dienolates, β-phosphonium enoates, and vinyl phosphonium ylides as intermediates. When these reactive zwitterionic species react with nucleophiles and electrophiles, they may generate carbo- and heterocycles with multifarious molecular architectures. This article describes the reactivities of these phosphonium zwitterions, the applications of phosphine catalysis in the syntheses of biologically active compounds and natural products, and recent developments in the enantioselective phosphine catalysis.

 Please wait while we load your content...
Please wait while we load your content...