Synthetic modification of salinomycin: selective O-acylation and biological evaluation†
Abstract
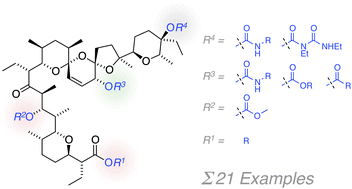
Salinomycin has found renewed interest as an agent for prevention of cancer recurrence through selectively targeting cancer stem cells. Strategies for generation of improved salinomycin analogs by individual modification of its hydroxyl groups are presented. An evaluation of the dose–response effects of the resulting library on breast cancer cell lines shows that acylation of the C20 hydroxyl can be used to improve IC50 values down to one fifth that of salinomycin.

 Please wait while we load your content...
Please wait while we load your content...