Enantioselective total synthesis of (+)-brazilin, (−)-brazilein and (+)-brazilide A†
Abstract
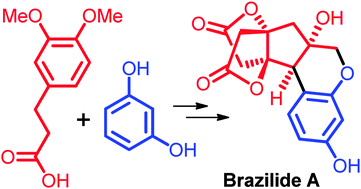
An enantioselective strategy for the synthesis of (+)-brazilin, (−)-brazilein and (+)-brazilide A has been developed. A Lewis acid mediated lactonization established the novel fused bis-lactone core of brazilide A and finalized the first total synthesis of (+)-brazilide A.

 Please wait while we load your content...
Please wait while we load your content...