Abstract
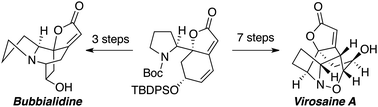
The first enantioselective total syntheses of virosaine A and bubbialidine are described. Key transformations include the formation of a tetracyclic intermediate via an intramolecular aza-Michael addition, generation of a N-hydroxy-pyrrolidine through a Cope elimination and an intramolecular [1,3]-dipolar cycloaddition to generate a complex 7-oxa-1-azabicyclo[3.2.1]octane ring system.

 Please wait while we load your content...
Please wait while we load your content...