Pyridyl- and benzimidazole-based ruthenium(iii) complex for selective chloride recognition through fluorescence spectroscopy†
Abstract
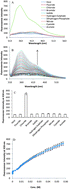
Three ruthenium(II) and (III) metal complexes of the compositions ([Ru(1)(Cl)(PPh3)]+ (4), ([Ru(2)(Cl)(PPh3)]+ (5) and ([Ru(3)(Cl)(H2O)]2+ (6) have been synthesized from oligodentate pyridyl-(1) and benzimidazole ligands (2–3) and structurally characterized. Our investigations revealed that if the coordination sphere of the metal ion carries a strong coordinating PPh3 ligand (complexes 4 and 5), the framework was not suitable for anion binding at all. However, when coordination of solvent (H2O) to the metal centre occurred instead (complex 6), a receptor for anion recognition through fluorescence spectroscopy could be generated. This allowed us to develop a sensor for the selective recognition of chloride over several other anions. In order to screen potential applications, the sensor was tested for the determination of chloride concentration in a series of four commercially available oral rehydration salts, giving excellent agreement with the values reported by the corresponding manufacturer. The detection limit was established to be 5 μM.

 Please wait while we load your content...
Please wait while we load your content...