Affinity analysis between immobilized l-arginine and plasmid isoforms provided by surface plasmon resonance†
Abstract
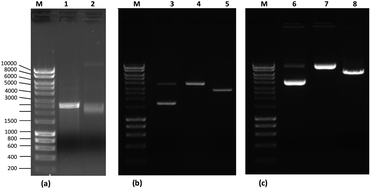
A strategy for studying the binding mode of plasmid isoforms to an immobilized amino acid as a model support has been developed using Surface Plasmon Resonance (SPR). The binding of L-arginine to plasmid isoforms of pGL101, pUC19 and pVAX1-LacZ (supercoiled, open-circular and linear) is examined by measuring the equilibrium dissociation constants (KD). L-Arginine was immobilized on a CM5 sensor chip and bound to plasmid isoforms with similar SPR binding profiles (square-shaped) and binding affinities ranging between 10−4 and 10−9 M. There are significant differences in the apparent affinity that is correlated with the three plasmids (2.39, 2.69 and 6.05 kbp). pGL101 shows the highest binding on the arginine surface followed by pVAX1-LacZ, while pUC19 shows the lowest binding. For the three plasmid isoforms, the supercoiled ones have the higher binding affinity to the arginine surface. Different buffer environments affect the interaction strength with an increase in response for Tris–HCl and a marked decrease for high salt concentrations. The knowledge of the affinity parameters is expected to provide further insights into the effect of plasmid topology on the purification by affinity chromatography.

 Please wait while we load your content...
Please wait while we load your content...