Analysis of intracellular enzyme activity by surface enhanced Raman scattering†
Abstract
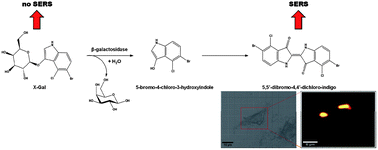
Dysfunctional intracellular enzymatic activity is believed to be an underlying cause of a myriad of diseases. We present the first use of surface enhanced Raman scattering (

 Please wait while we load your content...
Please wait while we load your content...