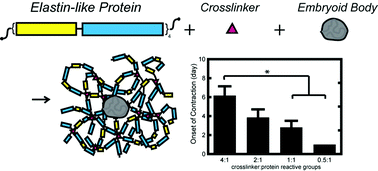
Systematically tunable in vitro platforms are invaluable in gaining insight to stem cell–microenvironment interactions in three-dimensional cultures. Utilizing recombinant protein technology, we independently tune hydrogel properties to systematically isolate the effects of matrix crosslinking density on cardiomyocyte differentiation, maturation, and function. We show that contracting human embryonic stem cell-derived cardiomyocytes (hESC-CMs) remain viable within four engineered elastin-like hydrogels of varying crosslinking densities with elastic moduli ranging from 0.45 to 2.4 kPa. Cardiomyocyte phenotype and function was maintained within hESC embryoid bodies for up to 2 weeks. Interestingly, increased crosslinking density was shown to transiently suspend spontaneous contractility. While encapsulated cells began spontaneous contractions at day 1 in hydrogels of the lowest crosslinking density, onset of contraction was increasingly delayed at higher crosslinking densities for up to 6 days. However, once spontaneous contraction was restored, the rate of contraction was similar within all materials (71 ± 8 beats per min). Additionally, all groups successfully responded to electrical pacing at both 1 and 2 Hz. This study demonstrates that encapsulated hESC-CMs respond to 3D matrix crosslinking density within elastin-like hydrogels and stresses the importance of investigating temporal cellular responses in 3D cultures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...