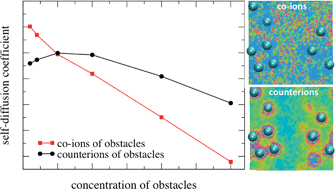
Self-diffusion coefficients of ions of a charge and size symmetric +1 : −1 electrolyte were studied in the presence of ionic obstacles carrying ten negative elementary charges within a “soft” version of the primitive model of electrolytes. The Brownian dynamics method was used to calculate the diffusion coefficients of mobile species. Simulations were complemented with the replica Ornstein–Zernike theory for partly-quenched systems to calculate the activity coefficients of individual ions and the Donnan exclusion coefficient. We study the influence of (i) the size of the pores, which varies with the concentration of quenched charged nanoparticles, and (ii) the presence of added salt on the mobility and thermodynamics of small ions. We show that for dilute matrices (low concentration of charged obstacles) the diffusion coefficient of counterions is smaller or equal to that of co-ions. The opposite is true for concentrated matrices: here counterions are considerably faster than co-ions. In dilute matrices, where obstacles are distributed at larger distances, the counterions are strongly attracted by fixed obstacles. As a consequence, they are slowed down in comparison with the co-ions. In concentrated matrices, where the average size of the pores is small, the counterions can take advantage of hopping from one obstacle to another, whereas the co-ions, repelled by the obstacles, need to diffuse in the space between the layers of counterions surrounding the obstacles. Thermodynamic calculations are consistent with the Brownian dynamics results.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...