Fusarisetin A: scalable total synthesis and related studies†
Abstract
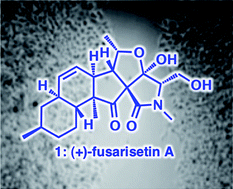
Fusarisetin A (1) is a recently isolated natural product that displays an unprecedented chemical motif and remarkable bioactivities as a potent cancer migration inhibitor. We describe here our studies leading to an efficient and scalable total synthesis of 1. Essential to the strategy was the development of a new route for the formation of a trans-decalin moiety of this compound and the application of an oxidative radical cyclization (ORC) reaction that produces fusarisetin A (1) from equisetin (2) via a bio-inspired process. TEMPO-induced and metal/O2-promoted ORC reactions were evaluated. Biological screening in vitro confirms the reported potency of (+)-1. Importantly, ex vivo studies show that this compound is able to inhibit different types of cell migration. Moreover, the C5 epimer of (+)-1 was also identified as a potent cancer migration inhibitor, while (−)-1 and 2 were found to be significantly less potent. The optimized synthesis is applicable on gram scale and provides a solid platform for analogue synthesis and methodical biological study.

 Please wait while we load your content...
Please wait while we load your content...