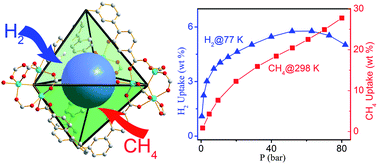
A 6,8-connected 3-dimensional metal–organic framework (2) of the tph topology was constructed from a new aromatic-rich, tetraphenylmethane-derived octa-carboxylate bridging ligand and trizinc cluster secondary building units (SBUs), and exhibited exceptionally high hydrogen (58 mg g−1 and 39 g L−1 at 52 bar and 77 K) and methane (276 mg g−1 and 189 g L−1 at 80 bar and 298 K) uptake capacities. 2 has the highest hydrogen uptake at 298 K among all of the MOFs that have been examined to date. The gravimetric and volumetric methane uptake capacities for 2 are the highest among thousands of MOFs that have been evaluated. We attribute the exceptionally high gas uptake capacities of 2 to the highly branched, aromatic-rich nature of the bridging ligand, optimal pore size, and the open metal sites in the trizinc SBUs in a stable high-connectivity MOF.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...