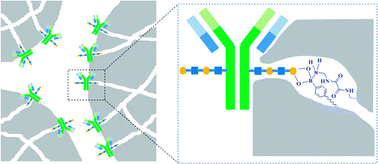
Antibodies are molecular workhorses in biological research, disease treatment and diagnostics. Purity is a critical prerequisite for antibody applications. Although protein A-based affinity chromatography has developed into the gold standard for antibody purification, protein A is associated with several apparent disadvantages, including high cost, poor stability and harsh product release conditions. Many attempts have been made towards molecular level biomimetics of protein A. However, practical substitutes have not yet been achieved. Here we present a novel functionalized material, called restricted access boronate affinity porous monolith, as a mimic of protein A for the specific capture of antibodies. This biomimetic relies on a novel strategy that combines the steric hindrance of the porous monolith with the chemical selectivity of boronic acid. This protein A biomimetic material demonstrated high specificity for antibodies. Meanwhile, original immunoaffinity and specificity of the captured antibodies were maintained. Compared with protein A, the monolithic biomimetic exhibited several significant advantages, including low cost, high stability and fast elution kinetics.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...