Imidazole-derived carbenes and their elusive tetraazafulvalene dimers†
Abstract
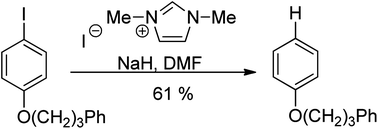
Previous efforts to prepare tetraazafulvalenes derived from imidazolium salt precursors have met with little success (one anomalously favourable example is known), and this is in line with the predicted reactivity of these compounds. However, we now report the preparation of a series of these tetraazafulvalenes formed either by deprotonation of 1,3-dialkylimidazolium salts or by Birch reduction of biimidazolium salts. The tetraazafulvalenes are highly reactive; for example, they act as Super-Electron-Donors towards iodoarenes. The two most reactive examples are formed more efficiently by Birch reduction than by the deprotonation route. Nevertheless, even in cases where the deprotonation approach affords a low stationary concentration, the mixture of precursor salt and base still produces the same powerful reductive chemistry that is the hallmark of tetraazafulvalene electron donors.

 Please wait while we load your content...
Please wait while we load your content...