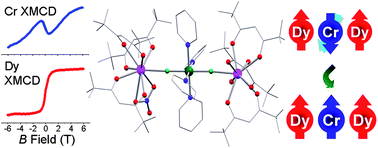
We report on the synthesis, crystal structure and magnetic characterisation of the trinuclear, fluoride-bridged, molecular nanomagnet [Dy(hfac)3(H2O)–CrF2(py)4–Dy(hfac)3(NO3)] (1) (hfacH = 1,1,1,5,5,5-hexafluoroacetylacetone, py = pyridine) and a closely related dinuclear species [Dy(hfac)4–CrF2(py)4]·½CHCl3 (2). Element-specific magnetisation curves obtained on 1 by X-ray magnetic circular dichroism (XMCD) allow us to directly observe the field-induced transition from a ferrimagnetic to a ferromagnetic arrangement of the Dy and Cr magnetic moments. By fitting a spin-Hamiltonian model to the XMCD data we extract a weak antiferromagnetic exchange coupling of j = −0.18 cm−1 between the DyIII and CrIII ions. The value found from XMCD is consistent with SQUID magnetometry and inelastic neutron scattering measurements. Furthermore, alternating current susceptibility and muon-spin relaxation measurements reveal that 1 shows thermally activated relaxation of magnetisation with a small effective barrier for magnetisation reversal of Δeff = 3 cm−1. Density-functional theory calculations show that the Dy–Cr couplings originate from superexchange via the fluoride bridges.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...