Base promoted highly efficient copper fluorapatite catalyzed coupling of phenols with arylboronic acids under mild and ligand-free conditions
Abstract
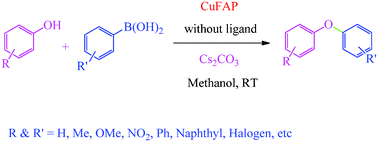
A mild, general and highly efficient protocol has been developed for the synthesis of diaryl ethers in good to excellent yield under mild and

 Please wait while we load your content...
Please wait while we load your content...